Research in the Evano Group 
The major part of our research is focused on the development of new processes in organic synthesis using copper-catalysis and copper-mediated transformations. More specifically, we have been involved in the use of copper-catalysis in natural and/or bioactive products, for the development of new processes in organic synthesis and ynamide chemistry and, more recently, in the development of new copper-catalyzed polymerization processes, all these research programs being strongly interconnected. Selected examples of these research activities are briefly described below.
![]() _Natural/bioactive products synthesis
_Natural/bioactive products synthesis
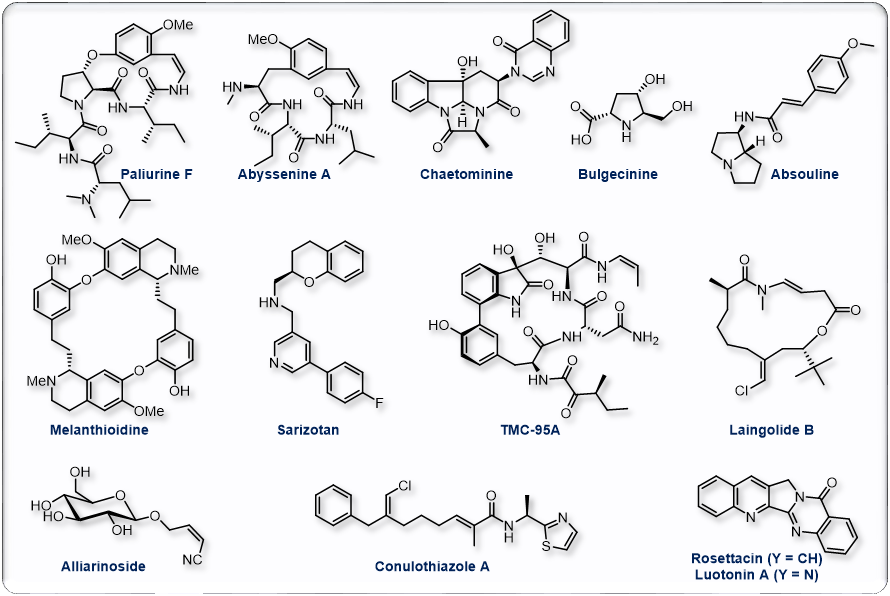
Natural products and total synthesis have long been a source of inspiration and motivation as well as considerable innovation and are one of the core of our research. They represent a unique opportunity for the fine-tuning of original techniques which have allowed us to gain access to the target molecules shown below or to their advanced precursors. Among other processes developed en route to these natural products, we have designed original copper-catalyzed functionalization, cyclization and macrocyclization reactions that enabled straightforward and original preparations of various alkaloids and other natural products .
Representative publications:
Org. Lett. 2005, 6, 5861; Angew. Chem. Int. Ed. 2007, 46, 572; J. Org. Chem. 2007, 72, 9003; J. Org. Chem. 2008, 73, 1270; Org. Lett. 2008, 10, 5027; Chem. Commun. 2009, 4166; Nat. Prod. Rep. 2013, 30, 1467; Org. Lett. 2014, 16, 1306; Chem. Commun. 2014, 50, 11907; Org. Lett. 2016, 18, 3542; Org. Lett. 2019, 21, 4318.
![]() _New processes in copper catalysis / copper organometallic chemistry
_New processes in copper catalysis / copper organometallic chemistry
We are equally involved in another research direction: copper catalysis and development of new copper-mediated transformations for organic synthesis. In this area, we have developed for example a new pathway to pyrrolindoles, a structural motif which is found in numerous natural or biologically active products, and designed various efficient processes for the synthesis of (heter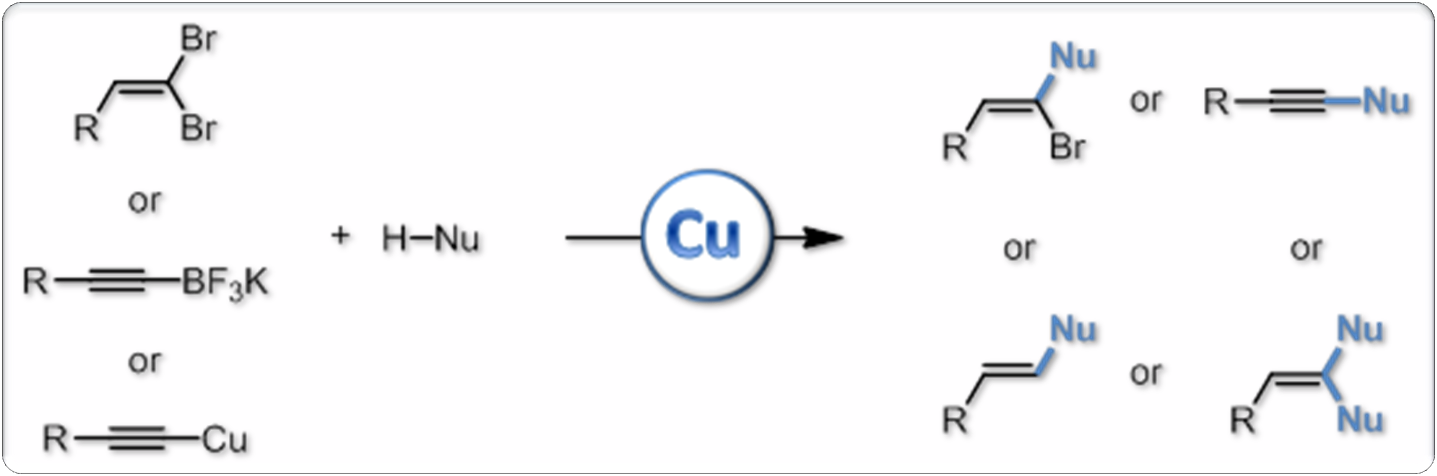 o)substituted alkenes and alkynes. More recently, we also have started to study the reactivity of stable organocopper reagents such as copper acetylides under oxidative conditions, which led to the development of efficient alkynylation procedures under especially mild and neutral conditions. These reactions we have developed are now commonly used in the group, as well as by other research groups, for the development of the chemistry of ynamides and for natural product synthesis as well.
o)substituted alkenes and alkynes. More recently, we also have started to study the reactivity of stable organocopper reagents such as copper acetylides under oxidative conditions, which led to the development of efficient alkynylation procedures under especially mild and neutral conditions. These reactions we have developed are now commonly used in the group, as well as by other research groups, for the development of the chemistry of ynamides and for natural product synthesis as well.
Representative publications:
Chem. Rev. 2008, 108, 3054; Org. Lett. 2008, 10, 3841; Angew. Chem. Int. Ed. 2009, 48, 4381; Org. Lett. 2009, 11, 4454; Org. Synth. 2010, 87, 231; Org. Lett. 2010, 12, 3272; Chem. Comm. 2011, 47, 179; Org. Lett. 2012, 14, 6; Chem. Sci. 2012, 3, 756; Org. Lett. 2012, 14, 1652; Organometallics 2012, 31, 7933; Org. Lett. 2013, 15, 4592; Adv. Synth. Catal. 2014, 356, 2051; Chem. Commun. 2014, 50, 10008; Org. Lett. 2014, 16, 4488; Chem. Commun. 2014, 50, 11907; ChemCatChem 2016, 8, 1319; Org. Lett. 2016, 18, 1438; Org. Lett. 2016, 18, 1904; Org. Synth. 2016, 93, 163; Org. Lett. 2017, 19, 6276; J. Org. Chem. 2019, 84, 392; Org. Process. Res. Dev. 2020, 24, 713; Angew. Chem. Int. Ed. 2021, 60, 21988.
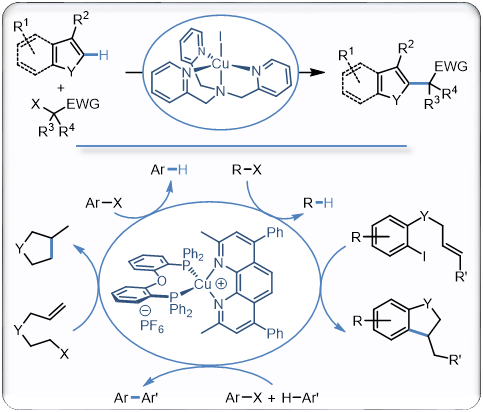 In addition to the processes mentioned above, we are also invoved in the use of copper complexes to catalyze the formation of radical species under mild and tin-free conditions. In this perspective, two type of complementary approaches have been developped The first one is based on a thermal activation with copper/polyamines and copper/polyimines complexes enabling the generation of radicals from activated alkyl halides and efficiently catalyzing the direct alkylation of a broad range of heteroarenes or carbonylative cross coupling reactions with carbon monoxide. The second approach is based on a photoactive complex simply activated with blue light and being a remarkable catalyst for a broad range of radical processes that readily proceed at room temperature. We are now using these efficient reactions in natural product synthesis while developping second generations catalysts based on these results and extending the copper-catalyzed generation of radicals to other processes in which other methods for the generation of radicals fail...
In addition to the processes mentioned above, we are also invoved in the use of copper complexes to catalyze the formation of radical species under mild and tin-free conditions. In this perspective, two type of complementary approaches have been developped The first one is based on a thermal activation with copper/polyamines and copper/polyimines complexes enabling the generation of radicals from activated alkyl halides and efficiently catalyzing the direct alkylation of a broad range of heteroarenes or carbonylative cross coupling reactions with carbon monoxide. The second approach is based on a photoactive complex simply activated with blue light and being a remarkable catalyst for a broad range of radical processes that readily proceed at room temperature. We are now using these efficient reactions in natural product synthesis while developping second generations catalysts based on these results and extending the copper-catalyzed generation of radicals to other processes in which other methods for the generation of radicals fail...
Representative publications:
Org. Lett. 2016, 18, 1438; Chem. Sci. 2017, 8, 3465; Org. Lett. 2017, 19, 3576; Synthesis 2018, 50, 3022; JoVE 2019, e59739; SynOpen 2021, 2, 141; Chem. Eur. J. 2022, 28, e202103599; Chem. Eur. J. 2022, 28, e202201356; Nat. Commun. 2022, 13, 560.
![]() _Chemistry of ynamides and reactive intermediates
_Chemistry of ynamides and reactive intermediates
We have started in 2009 a research program aimed at the development of the chemistry of ynamides, most useful building blocks in organic synthesis whose chemistry is under intense exploration. In order to bring significant developments in this area, we have studied the chemistry of ynamides using a global approach involving the development of new and general ynamide syntheses, the design of new transformations with these building blocks and a combined theoretical/experimental approach to better apprehend their reactivity. In this context, we have reported three generations of ynamides syntheses based on copper-mediated transformations with dibromoalkenes, potassium alkynyltrifluoroborates and copper acetylides. These complementary routes allow the preparation of most classes of ynamides and are routinely used on a multigram scale in the lab (and teaching lab as well). We have also developed new processes with ynamides such as, for examples, new syntheses of dihydropyridines, pyridines, aminovinylphosphonates, fluoroenamides, selectively deuterated amines or complex polycyclic molecular scaffolds.
ynamides, most useful building blocks in organic synthesis whose chemistry is under intense exploration. In order to bring significant developments in this area, we have studied the chemistry of ynamides using a global approach involving the development of new and general ynamide syntheses, the design of new transformations with these building blocks and a combined theoretical/experimental approach to better apprehend their reactivity. In this context, we have reported three generations of ynamides syntheses based on copper-mediated transformations with dibromoalkenes, potassium alkynyltrifluoroborates and copper acetylides. These complementary routes allow the preparation of most classes of ynamides and are routinely used on a multigram scale in the lab (and teaching lab as well). We have also developed new processes with ynamides such as, for examples, new syntheses of dihydropyridines, pyridines, aminovinylphosphonates, fluoroenamides, selectively deuterated amines or complex polycyclic molecular scaffolds.
Representative publications:
Angew. Chem. Int. Ed. 2009, 48, 4381; Angew. Chem. Int. Ed. 2010, 49, 2840; Org. Synth. 2010, 87, 231; Org. Lett. 2010, 12, 3272; Adv. Synth. Catal. 2011, 353, 263; Org. Lett. 2012, 14, 6; Chem. Sci. 2012, 3, 756; Org. Lett. 2012, 14, 1652; Organometallics 2012, 31, 7933; Chem. Commun. 2012, 48, 5196; J. Am. Chem. Soc. 2012, 134, 9078; Org. Lett. 2013, 15, 3122; Angew. Chem. Int. Ed. 2014, 53, 4968; Org. Lett. 2014, 16, 2252; J. Am. Chem. Soc. 2014, 136, 12528; Aldrichimica Acta 2015, 48, 59; J. Org. Chem. 2015, 80, 3397; Angew. Chem. Int. Ed. 2016, 55, 4547; Synthesis 2018, 50, 3022; Angew. Chem. Int. Ed. 2020, 59, 242; Chem. Sci. 2021, 12, 1157; Nat. Commun. 2022, 13, 560; ACS Org. Inorg. Au 2022, 2, 53.
Some ynamides from our lab are now available from Merck: Ynamides: Versatile and Enabling Building Blocks for Chemical Synthesis (sigmaaldrich.com) & Evano Group – Professor Product Portal (sigmaaldrich.com)
For our Technology Spotlight on Ynamides, click here; For the group webpage on the Sigma-Aldrich Professor Product Portal, click here.
![]() _Polymers
_Polymers
This research program has recently started and aims at the development of new, mild and efficient polymerization processes based on copper catalysis and copper organometallic chemistry and on the development of sustainable polymerization and depolymerization processes.
