Chemistry World: Introducing iteroselectivity - A new concept to better describe some chemical reactions by R. Lavendomme and I. Jabin - 8 May 2023
Actus ULB: L’itérosélectivité: la sélectivité qui manquait à la chimie organique.
Actus ULB: Des tests de diagnostic rapide plus efficaces grâce aux nanoparticules d’argent.
Actus ULB - Grand angle: Un nouveau test de dépistage rapide.
ULB Technology Transfer Office: Levée de fonds pour X4C, une spin-off de l'ULB.
L'Echo: La spin-off X4C propose d'améliorer les tests diagnostiques.
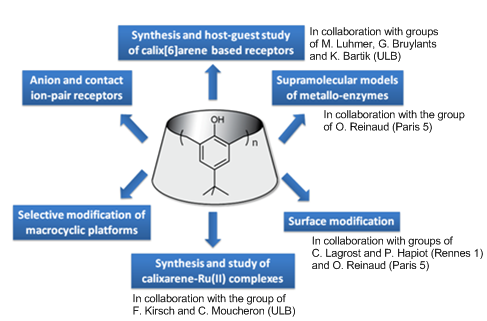
Our research interests are at the interface of organic synthesis and supramolecular chemistry. In particular, we are interested in the design, synthesis and study of novel molecular receptors derived from macrocyclic platforms such as calixarenes as well as the use of calixarenes for surface modification. Rational synthetic strategies for the selective modification of macrocyclic platforms as well as the host-guest properties of calix[6]arene-based receptors toward charged or neutral species are investigated. These receptors are notably applied in the sensing of anions, ion-pairs, metal ions or bioactive ammonium ions and in the design of biomimetic metallo-receptors.
Synthesis and host-guest study of calix[6]arene-based receptors
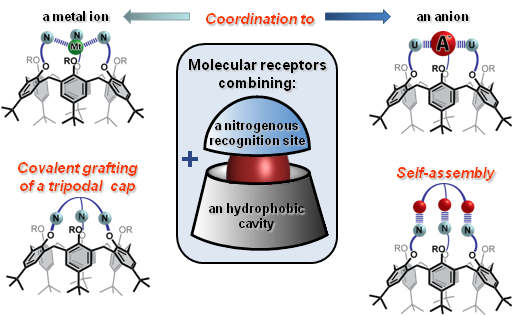
Calix[n]arenes are concave macrocyclic structures possessing an hydrophobic cavity that can accommodate charged or neutral species. Among the different oligomers, calix[4]arenes have been extensively studied but they suffer from the smallness of their cavity and consequently they have been mostly used as molecular platforms for the preorganization of a binding site outside of the cavity. In contrast, the larger calix[6]arenes display a cavity which is well adapted for the inclusion of organic guests. However, their high conformational flexibility has first to be restricted in order to obtain a well defined cavity suitable for host-guest chemistry applications. For this, we have developed different strategies such as (i) the grafting of covalent bridges, (ii) coordination to an anion or a metal-ion, (iii) self-assembly or (iv) the introduction of bulky groups (e.g. Boc). Different families of calix[6]arene-based receptors have been described the last 10 years: the calix[6]azacryptands, tubular bis-calix[6]arenes, calix[6]arene bearing coordinating arms for anions or metal ions, self-assembled systems, etc. Most of these receptors display remarkably selective and versatile host-guest properties toward charged (ammonium ions, metal ions, anions, ion-pairs) or neutral species.
Strategies for the rigidification of calix[6]arene-based receptors.

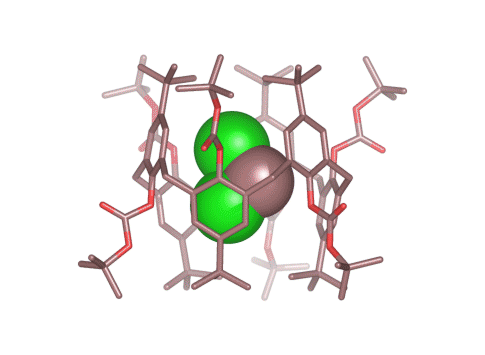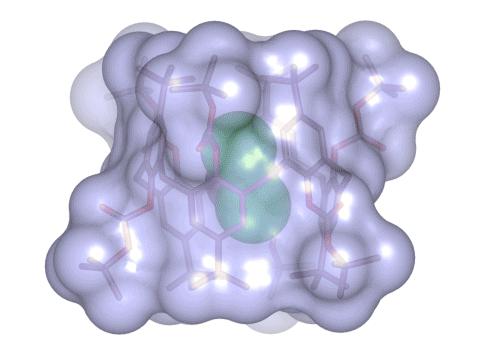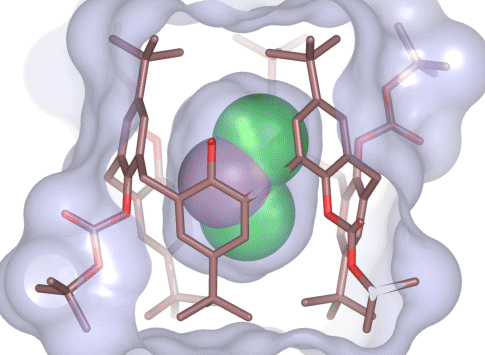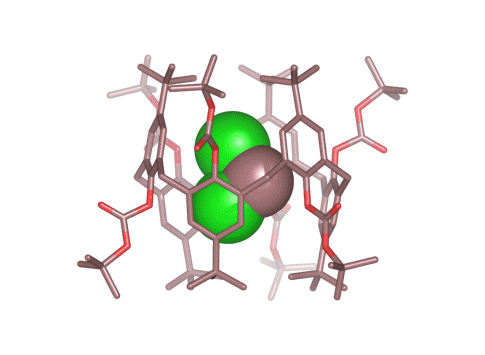
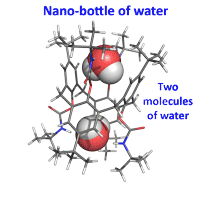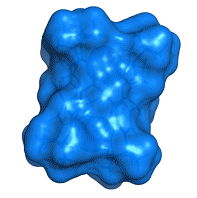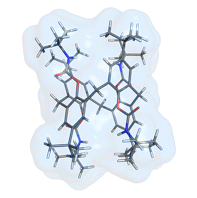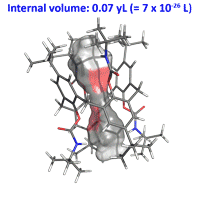
Calix[4 and 6]arene-based molecular containers encapsulating small molecules .
Representative publications:
Anions, ammonium ions, zwitterions, contact ion-pairs and ion-triplets receptors
We have reported different calix[6]arene based receptors that combine a donor binding site (composed of either amido, ureido or thioureido groups) in close proximity to the hydrophobic calixarene cavity. These calix[6]arenes based systems are constrained in the cone conformation either through coordination to an anion or covalent capping. This leads to several families of anions, ammonium ions,contact ion-pairs and zwitterions receptors.
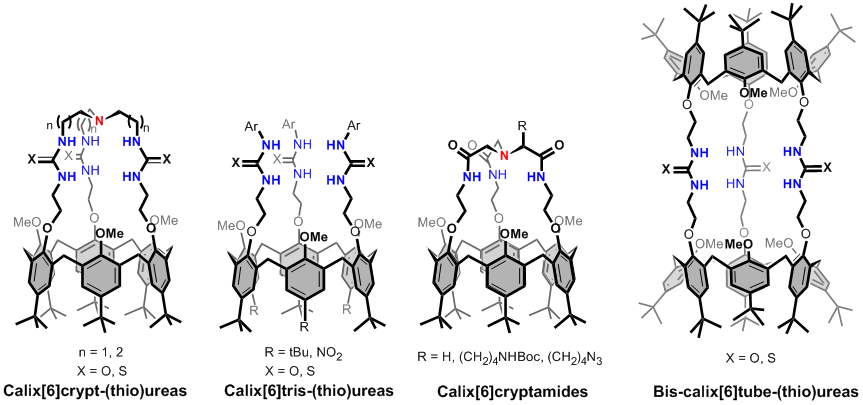
Different families of anions and contact ion-pairs calix[6]arene based receptors.
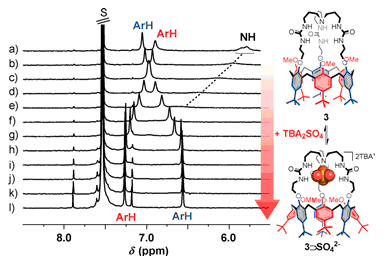
One of these families (i.e. the calix[6]tris-ureas) can strongly bind either anions or organic contact ion-pairs in a cooperative way. Indeed, the converging ureido groups allow strong binding to anions, which, in turn, allows strong binding of an ion-paired ammonium accommodated into the calixarene cavity. Even more versatile heteroditopic receptors have been obtained through the grafting of a covalent tris-ureido or tris-amido cap on the narrow rim of the calix cavity (calix[6]cryptureas and calix[6]cryptamides). These neutral receptors exhibit unique host-guest properties toward polar neutral molecules (e.g. ureas, amides), anions, ammonium ions or contact organic ion-pairs even in a protic environment. Within each family of guests, these receptors are able to discriminate between different guests with a high degree of selectivity. For example, calix[6]cryptamides can selectively bind contact ammonium fluoride salts through a highly cooperative process. Moreover, it has been shown that the versatile host properties of all these receptors can be controlled by protonation of the apical nitrogen of the basic tripodal cap. Thus, guest release triggered by the addition of acid or sophisticated supramolecular switches based on the acid/base interconversion of host-guest systems have been reported with these receptors.
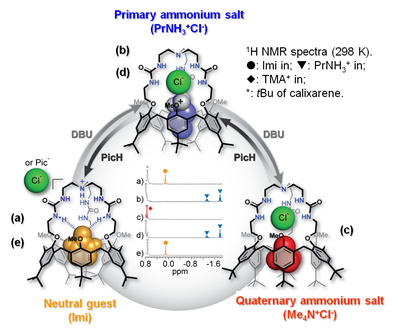 |
 |
|
Example of three-way supramolecular switch |
Exemple of anion titration monitored by 1H NMR spectroscopy. |
The protonation of the cap may also be used for the reinforcement of the binding properties (toward neutral guests) and for the development of rare examples of metal-free cascade complexes even stable in a protic environment. Moreover, the efficient recognition of biologically relevant ammonium ions (e.g. acetylcholine, dopamine) has been achieved in a protic environment.
Finally, bis-calix[6]tube-(thio)ureas are D3h-symmetric tail-to-tail bis-calix[6]arene featuring two divergent cavities triply connected by (thio)ureido linkages. These receptors are especially efficient for the complexation of organic ion triplets even in a protic environment. The resulting quaternary complexes represent rare examples of cascade complexes with organic cations.
![calix[6]tube](/images/stories/research/tube-bisEtNH3SO4-7.bmp) |
![Animated calix[6]tube](/images/stories/research/tube%20bis%20dodecylamonium%20sulfate.gif) |
|
XRD structure of a bis-calix[6]tube-urea including an ion triplet, |
Chem3D model of a bis-calix[6]tube-urea including an ion triplet, i.e. (CH3(CH2)11NH3+)2SO42-. |
Representative publications:
Supramolecular models of metallo-enzymes
This research was initiated by the group of O. Reinaud (Paris 5) in the 90's and our two groups started to collaborate in 2000 (more than 30 joint articles in the field of calixarene chemistry).
Enzymes are fascinating catalysts. They carry out reactions with exquisite control of the chemical reactivity under mild conditions. One question naturally arises: how do they work? Unfortunately, these macromolecules are complex and difficult to study directly. Hence, many groups have synthesized simpler biomimetic models to study enzymatic behavior. This approach has proved to be very useful and has contributed to a better understanding of enzymatic mechanisms. In strong collaboration with the group of Pr. O. Reinaud, our group has been very active in this area. We are particularly interested by mononuclear zinc and copper metallo-enzymes (enzymes which contain a single zinc or copper metal ion at their active site). With the aim of designing such biomimetic complexes, we have developed a large family of calix[6]arene-based receptors (calix[6]azacryptands) that present a tripodal aza-cap which can bind a metal ion. Besides their binding properties toward metal ions, the calix[6]azacryptands display remarkably versatile host-guest properties toward neutral or charged guests (e.g. ammonium ions).

Structure of the different calix[6]azacryptants.
The tripodal aza-cap mimicks the poly-histidine core often encountered in the active site of a mononuclear metallo-enzyme. These compounds display a single coordination site accessible for an exogenous ligand whose binding is fully controlled by the calix[6]arene cavity. This approach is original since all metallo-enzyme models reported so far proposed a biomimetic coordination core next to a cavity but not in a cavity.
![Biomimetic calix[6]azacryptands](/images/stories/research/enzyme2.bmp)
A zinc metallo-enzyme (left) and one of our biomimetic model (right).
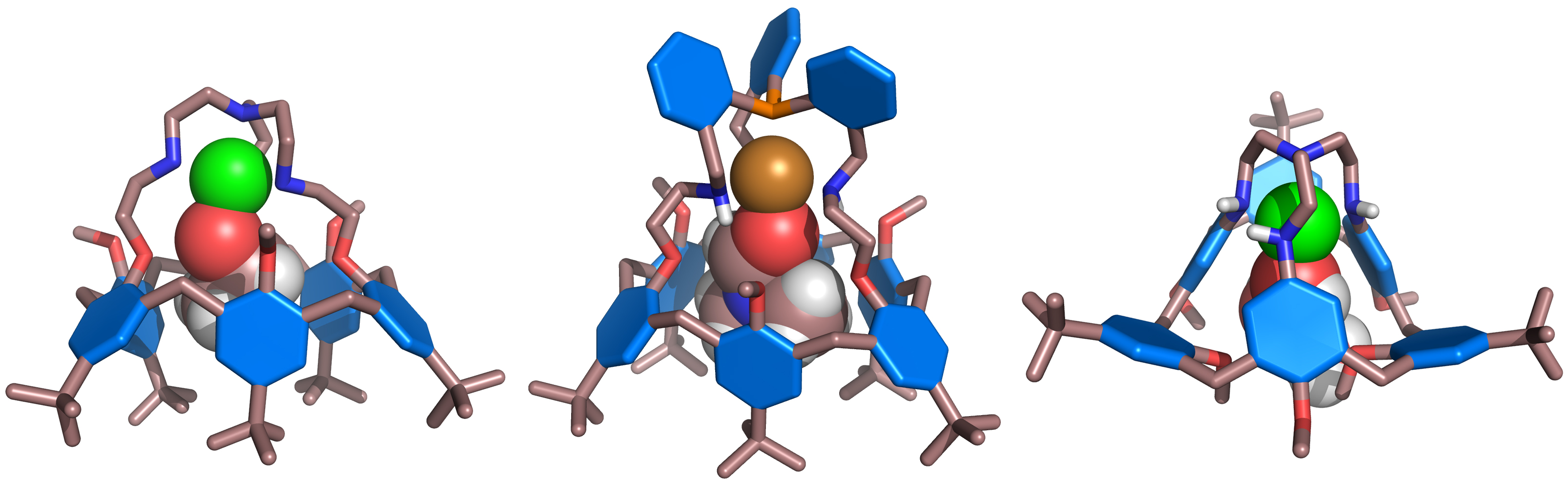
X-ray structures of various Zn or Cu calix[6]arene-based complexes hosting neutral guests.
The corresponding Zn2+ and Cun+ complexes have been the subject of intense studies in solution, either spectroscopically, or by electrochemical means (in the case of the Cun+ complexes) which showed that they indeed provide accurate mimicry for selective substrate binding and guest-controlled redox properties. Moreover, catalytic properties could be evidenced with calix[6]tren and -tmpa copper(I) complexes.
This research is developed in strong collaboration with three other groups: the group of Prof. O. Reinaud (LCBPT, UMR CNRS 860, Université Paris Descartes) the group of Dr. Y. Le Mest (CEMCA, UMR CNRS 6521, Université de Bretagne Occidentale) and the group of Dr. P. Hapiot and Dr. C. Lagrost (MaCSE, UMR CNRS 6226, Université de Rennes 1).
Representative publications:
|
|
Selective modification of macrocyclic platforms
Selective functionalization of macrocycles is a key issue. Notably, the selective modification of cavity-based receptors can confer them new properties (enhanced recognition properties, hydrosolubility, fluorescence, chirality, etc.) and thus is usually required for the development of applications (design of sensors, catalysts, etc.). However, to achieve highly selective reactions involving oligomeric platforms is still a challenge. Indeed, the presence of multiple identical functions such as phenolic groups can lead to multiple unwanted by-products (regioisomers, etc.). Furthermore, the rationalization of the selectivity and the development of general methods is even more challenging. In this context, our group is involved in the development of rational and general methods for the selective functionalization of the small and large rims of calixarenes. Examples of recent strategies that we developed are shown below.
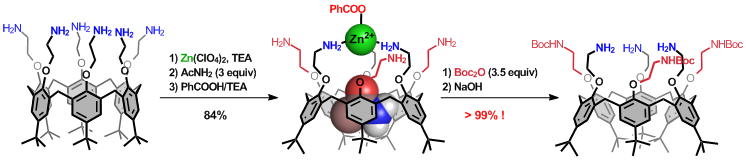
Highly selective 1,3,5-tris-protection thanks to host-guest and coordination chemistry.
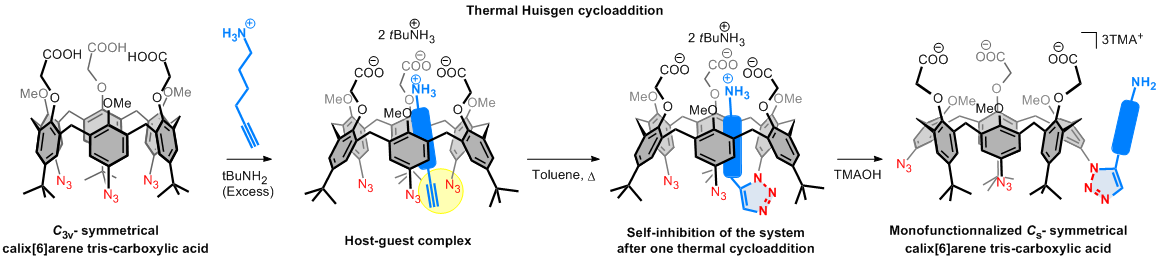
Supramolecular assistance for the selective mono-functionalization of a calix[6]arene tris-carboxylic acid receptor
(collab. with B. Colasson, Paris 5)
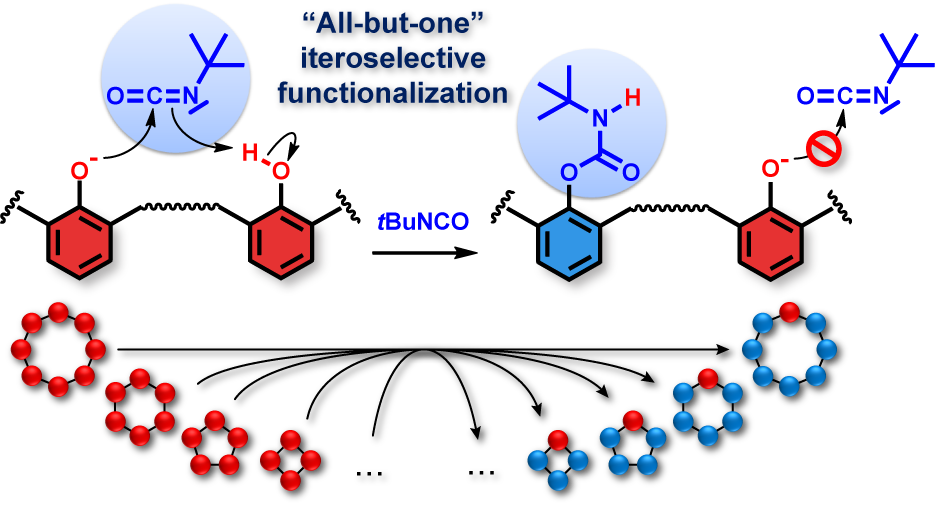
Highly selective and general methodology for the functionalization and protection of all but one phenol unit on calixarenes.
Representative publications:
Surface modification with calix[4]arenes
One essential issue in the development of materials presenting an accurately functionalized surface is to achieve control on layers structuring. Whereas the very popular method based on the spontaneous adsorption of alkanethiols on metal (SAMs) is facing problems of stability, the reductive electrografting of aryldiazonium salts yielding stable interface on various substrates, knocks against the difficult control of the formation and organization of monolayers. Recently, in the frame of a collaborative project (vide infra), we have developed a unique strategy that consists of the covalent grafting on the surface of calix[4]arene-tetradiazoniums bearing either alkyl, fluoroalkyl or functional groups (e.g. COOH, COCl, NHS-activated acid, alkyne, azide). This methodology provides:
-
well-organized, rather compact and robust monolayers that can serve either as pre-functionalized, hydrophobic or hydrophilic surfaces.
-
pre-functionalized platforms that can be further used for the direct immobilization of molecules, biomolecules (carbohydrates, DNA, proteins, etc.), nanoparticles, oligomers, … on any conducting or semiconducting surfaces. These pre-functionalized ready-to-use surfaces can be useful in a wide range of applications: (bio)sensors, electronic, optic, coating, biomedical, etc.
This research is developped in strong collaboration with the group of Dr. C. Lagrost and Dr. Y. Leroux (MaCSE, UMR CNRS 6226, Université de Rennes), the group of G. Bruylants (ZMNS, ULB) and the group of Prof. O. Reinaud (LCBPT, UMR CNRS 860, Université Paris Descartes).
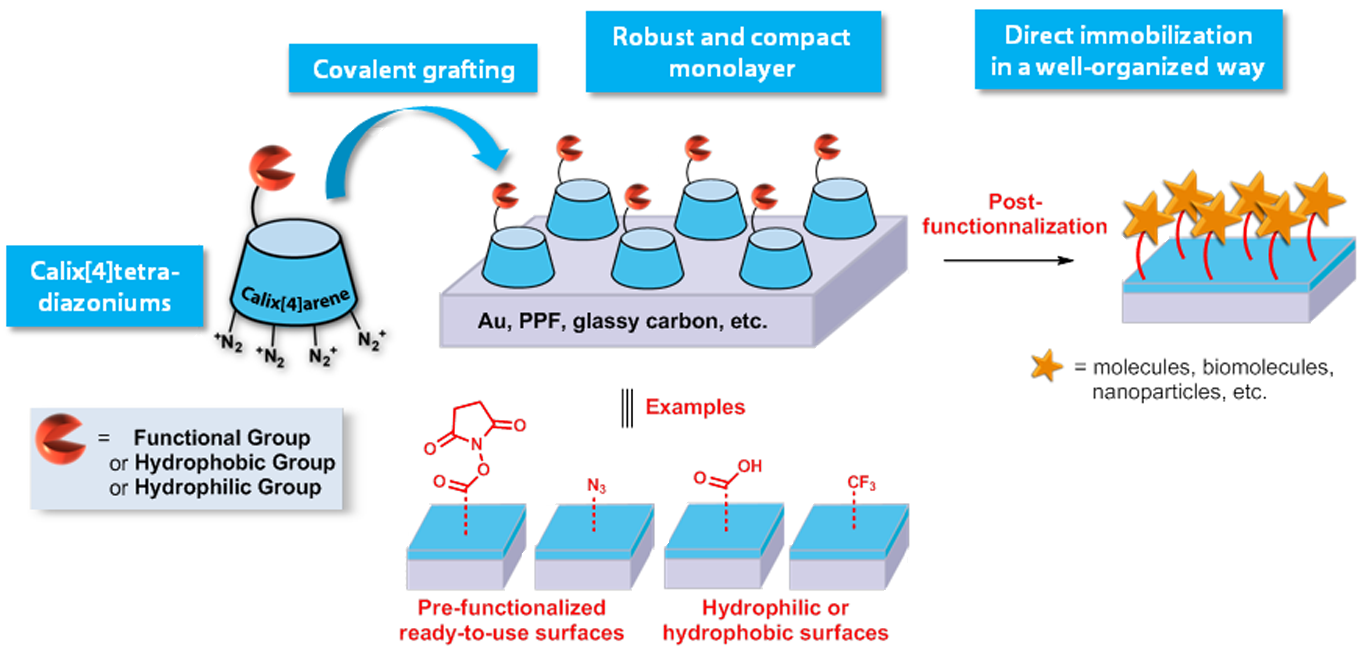
Methodology allowing surfaces modification in a well-organized way.
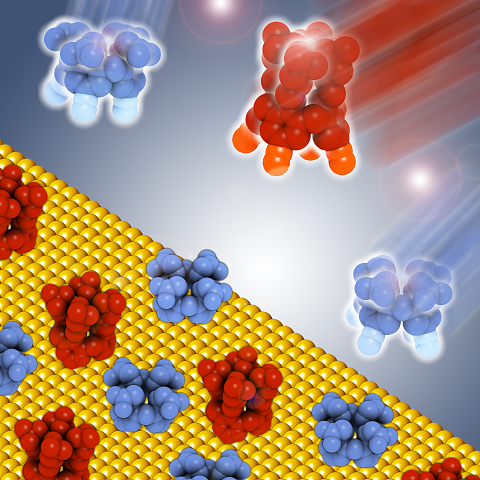
Mixed monolayer grafted on a surface with controlled composition.
Representative publications:
|
PCT/EP2013/056789, 28 March 2013
|